
Early Cancer Screening
Trucheck™ is a new blood-based paradigm in multicancer detection.
The following options are available:
- Trucheck™ intelli (up to 70 solid tumours)
- Trucheck™ FemmeSafe (Breast, Ovaries, Uterus, Cervix)
About Trucheck™
About 4.4 million new cancers are detected every year in Europe as well as about 2 million cancer related deaths. Unfortunately, some cancers are detected at advanced stages which necessitate more intensive and expensive treatments which have greater risk of side effects. Detection of cancers at early / local stages is vital for successful treatments, lower treatment costs, lower toxicities and improved survival. Trucheck™ is the culmination of years of collaborative international research and innovation and has been developed, tested and validated on > 40.000 individuals.
Trucheck™ technology
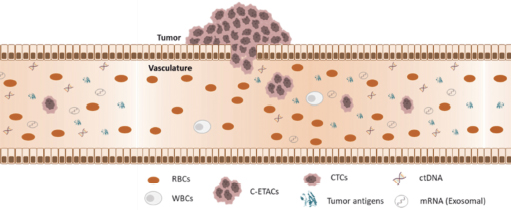
- Trucheck™ detects circulating tumour cells (CTCs) and clusters of these CTCs which are released by malignant tumours, but not from non-cancerous (normal / benign tumour / inflammatory) tissue. Hence CTCs are ubiquitously seen in blood of cancer patients but absent in blood of healthy individuals.
- Trucheck™ intelli can distinguish ~70 types of solid tumours which account for ~81% of all cancer cases and ~84% of all cancer related deaths in Europe.
- In clinical studies, Trucheck™ has a sensitivity of 88% in the detection of cancer at all stages and types. Further Trucheck™ intelli has an accuracy of 96% in determining the tissue or organ of origin in positive cases.
- Real-world data has shown a sensitivity, depending on the tumor, of 65% – 89% and a specificity of 96% – 99%.
- Trucheck™ detects cancers irrespective of the extent of the disease, thus even early-stage cancers are reliably observed.
Trucheck™ is particulary recommended for:
- Asymptomatic individuals who have a family history of cancer.
- Individuals who want to include this test in their yearly check-up.
- Asymptomatic individuals who have a high risk in cancer.
Important publications
Akolkar D. et al.
Circulating ensembles of tumor-associated cells: A redoubtable new systemic hallmark of cancer (International Journal of Cancer, 2020)
Population – Cancers: 5.509 (Retrospective) • Asymptomatic: 10.625 (Prospective)
Parameters – Analyte: C-ETACs, CTCs • Proof of Concept Study
Performance Characteristics – Specificity: 96.4% (Asymptomatic) • Sensitivity: 89.5% (Retrospective)
Renade A. et al.
Population – Cancers: 5.509 (Retrospective) & 4.419 (P) • Benign: 324 (Prospective) • Asymptomatic: 10.625 (Prospective)
Parameters – Analyte: C-ETACs, CTCs • Assessment: Colony Detection Assay • Proof of Concept Follow-up Study
Performance Characteristics – Specificity: 97.5% (Benign) & 95.6% (Asymptomatic) • Sensitivity: 93.0% (Retrospective) & 93.0% (Prospective)
Population – Cancers: 9.416 (Retrospective) & 6.025 (Prospective) • Benign: 700 • Asymptomatic: 13.919 (Prospective)
Parameters – Analyte: C-ETACs, CTCs • Assessment: Colony Detection Assay • Clinical Validation Study
Performance Characteristics – Specificity: 99.3% (Benign) & 100.0% (Asymptomatic) • Sensitivity: 85.2% (Retrospective) & 86.7% (Prospective)
Case-Control Study
Population – Breast Cancer Cases: 548 • Asymptomatic: 9.632
Parameter – Analyte: Breast-Adenocarcinoma-Associated CTCs
Performance Characteristics – Specificity: 100.0% • Sensitivity: 92.07%
Prospective clinical study
Population – Breast Cancer Cases: 112 • Benign Breast Conditions: 29
Parameter – Analyte: Breast-Adenocarcinoma-Associated CTCs
Performance Characteristics – Specificity: 93.1% • Sensitivity: 94.64%
Advantages
Method
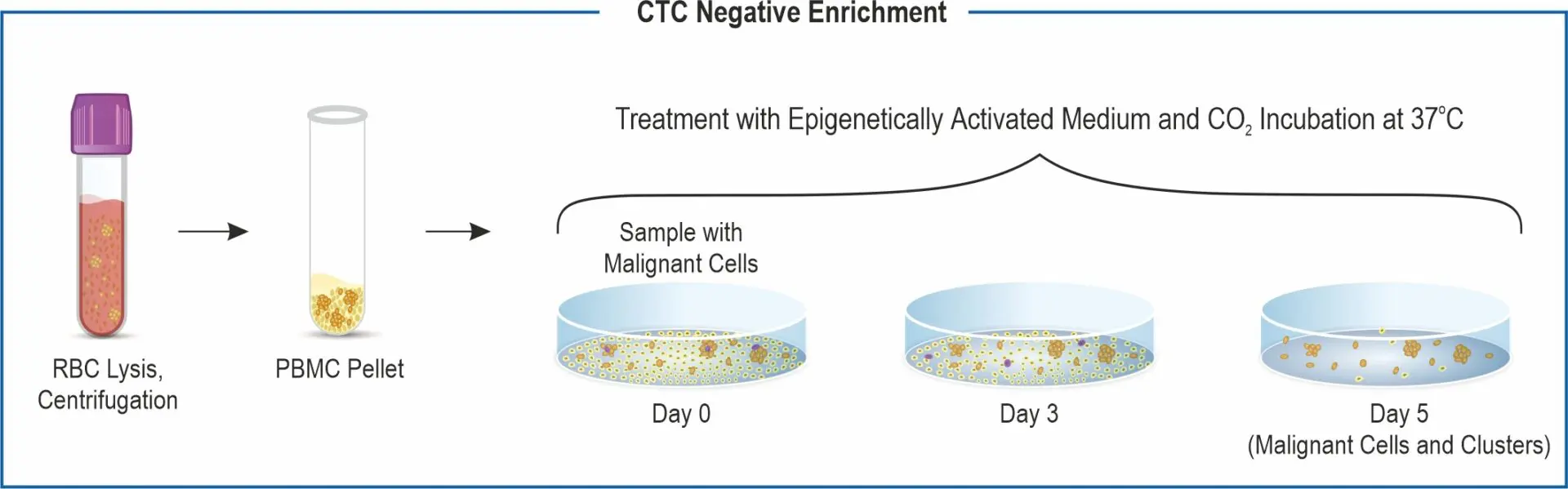
CTC enrichment:

Microscopic images of stained cells from a cancer patient
Sample collection
Requirements:
Blood draw:
3 EDTA tubes (purple colour cap) – 2 x 10 ml and 1 x 6 ml – in total: 26 ml
Note
Sequence of draw should not be altered. Blood draw should be performed only by qualified phlebotomist under medical supervision. Ship at +2 °C to +6 °C in the container provided by DCG.
Precautions:
- Blood sample must be collected following a 6 hour fasting period. Consumption of fluids is permitted.
- All 4 pages of the Test Request Form (TRF) are to be read and completed by the patient and the doctor. Please provide location, date and signature in all relevant places.
Turn Around Time
10 days from receipt of sample


